Molybdenum Disulfide Nanosheet - Mechanical Milling Petreatment
Introduction
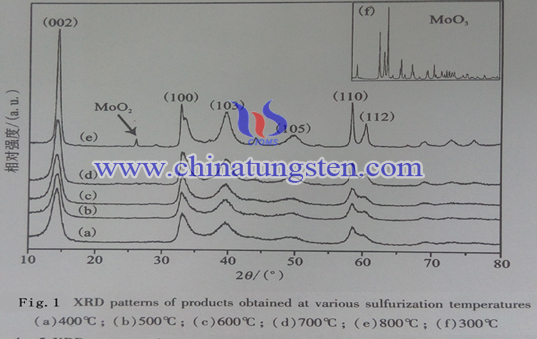
It can be seen from Figure 1, under the same experimental conditions, mix MoS3 which has not been ball milling pretreated with sulphur, only observe a little MoS2, and most products are MoO2, this just intermediate products of vulcanization [Figure 1 (c)]. The formation of molybdenum disulfide is a substitution and reduction reaction step, which is similar with oxidation of molybdenum trioxide, the former is a rapid reaction while the latter is diffusion controlled process determined by a slow diffusion rate. So, if it has not been milling, the particle size of the reactants would be too large, resulting in that it is impossible to complete its reduction rapidly, reduction should be completed through from outside to inside the diffusion of sulfur which would takes a long time, and even all the sulfur source have been volatized, there are only a small amount of sheet molybdenum disulfide.
However, if after ball milling pretreatment, the particle size would become small with a uniform distribution, which can increase the area of the direct contact of the reactants, so that the reaction time can be shortened (Fig. 2).
In the gas-solid reaction model, the size of the final product depends on the size of the original oxide nanoparticles. However, after mechanical milling pretreatment,the size of morphology of the final product also depends on sulfur. Because that after pretreatment, MoS3 and sulfur can achieve ultrafine uniform, intimate contact each other and form a micro reaction zone. At the begin of processing and high temperature calcination, they can quickly react in the reaction zone to generate MoS2 film, thereby preventing the molybdenum oxide core continues to grow, and then complete the reaction by diffusion vulcanization to obtain nanosheet molybdenum disulfide. Therefore, it can be concluded that a mechanical milling pretreatment plays a key role on the formation of molybdenum disulfide nanosheet, it also achieve a uniform distribution of ultrafine particles so as to form a plurality of "micro-reaction", effectively speed up the reaction rate, limiting the growth of the kernel.

